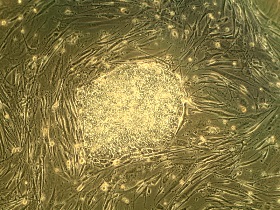
<p>Medical testing is the process of studying a new or revised medical treatment on humans. Initially new drugs are tested in laboratories using stem cell research. Stem cells provide an excellent testing medium at this stage of the development process because very little is know about the new treatment, or how it is metabolized. Stem cells provide a means to safely test the treatment and establish whether or not the compound is harmful. Since the most common reason for new drugs to fail during their development process is toxicity to the human liver, human embryonic stem cells are transformed into liver tissue prior to carrying out the trials.<br />
Late in 2007, a consortium of government backed pharmaceutical companies and scientists formed a UK stem cell bank to provide stem cells for medical research. The UK stem cell bank provides laboratory grade stem cells to pharmaceutical development companies for the purpose of conducting laboratory testing. Once a treatment has passed all stages of laboratory testing, it may then proceed to FIH (First In Human) Phase 1 Medical Testing, providing that the MHRA and an independent ethics committee approve the trials, both of whom will have been provided full access to the findings of the initial laboratory testing before reaching a decision.<br />
Before gaining the relevant licensing required for a treatment to be made available for prescription, the treatment must pass three phases of clinical trials. Phase I trials are carried out on a comparatively small number of people, they seek to establish the safe dosage range of the treatment, identify any side effects that the treatment may cause and evaluate the safety of the treatment. Phase II trials involve a larger group of people and continue the evaluation of a treatments safety, they also seek to establish how effective the new drug is at treating the symptoms it is intended to alleviate.<br />
Phase III trials can involve between one and three thousand people, they compare the effectiveness of the treatment against existing treatments and make further evaluations regarding the safety of the drug and observations related to its safe usage. These trials are not carried out by the company responsible for the development of the treatment, instead a specialist clinical trials company like Covance carry out the trials. This is because the MHRA, the UK&#8217;s Medicines and Health care products Regulatory Agency ultimately require unbiased trial results on which to base their licensing decision.<br />
Once a new treatment has been licensed it is effectively available for doctors to prescribe, and can be used to treat the ailments it was developed to alleviate. At this point Phase IV medial trials commence, since the treatment is actively available the study involves actual patients, and the test group is therefore far more expansive than those used in previous studies. Even upon the completion of phase IV trials the treatment does not become exempt from further scrutiny, because all treatments available in the UK are constantly monitored and tested by the MHRA throughout the lifetime of their availability.</p>
<h5>Featured images:</h5>
<ul>
<li> <span class="license">License: Image author owned</span></li>
</ul>
<p>This article was written by Nick Davison for Covance Leeds, a leading UK clinical trials company.</p>

What Is Medical Testing And Why Is It Required?